VARIOUS ENTITIES WILL MAKE UP THE CORE OF THE iREACH HEALTH ECOSYSTEM
STATE-OF-THE-ART BUILDINGS
iREACH Health will be centred in two state-of-the-art buildings beside Belfast City Hospital, which will open in 2027.
Site A (patient-facing clinical trial delivery) will include a 100-bed Celerion facility, research pharmacy units and 20 Northern Ireland Clinical Research Facility (NICRF) clinical rooms as well as a Research Pharmacy unit. This site will be located within the City Hospital, Belfast site, with a joining link to the BHSCT, facilitating access to clinical emergency services, to meet industry service-level requirements and drive early phase trials.
Site B (core space to support set-up and delivery of clinical trials) will accommodate clinical trial support infrastructure (HSC Research Office, HSC Innovations, Finance, HSC R&D), industry tenants (Celerion) and lab service space (Evolve Service Lab), creating a thriving infrastructure working together in collaboration with the surrounding clinical and academic community. It will also include an Innovation Hub and an interactive space for patient and public engagement.,

BUILDING A MORE CONNECTED HEALTHCARE RESEARCH ECOSYSTEM
iREACH Health is a state-of-the-art facility in Northern Ireland, bringing together industry, academia and Health and Social Care to transform the healthcare research ecosystem. Together, we will accelerate the discovery, development and early phase testing of new treatments, paving the way for rapid medical advancements. iREACH Health will maximise the potential of research, advance human health, support the creation of jobs, and promote inclusion and well-being to place people at the heart of research.
By connecting start-ups and pharmaceutical companies to an optimised ecosystem, iREACH Health creates a connected innovation landscape. iREACH Health will span basic research (discovery and pre-clinical), translation to humans (proof of concept), and translation to patients (clinical trials).

Celerion
iREACH Health's flagship industry tenant Celerion will take up residence in 3 floors of site A which will enable them to expend their operations in Belfast.
Celerion offers the following services
- Early Phase Clinical Research: Offers comprehensive early phase services, including First-in-Human (FIH) studies, bioavailability and bioequivalence studies, and dose-ranging studies (SAD/MAD)
Bioanalytical Sciences: Provides robust bioanalytical data for small and large molecules, including PK/PD, immunogenicity, and biomarker analysis - Data Management & Biometrics: Offers services in study design, data collection, analysis, and reporting, ensuring high-quality data and efficient study execution
- Patient Recruitment: Utilizes best-in-class recruitment strategies, including a large database of active participants, to ensure timely study enrollment
- Regulatory Affairs: Provides insights and support to navigate regulatory requirements and accelerate drug development
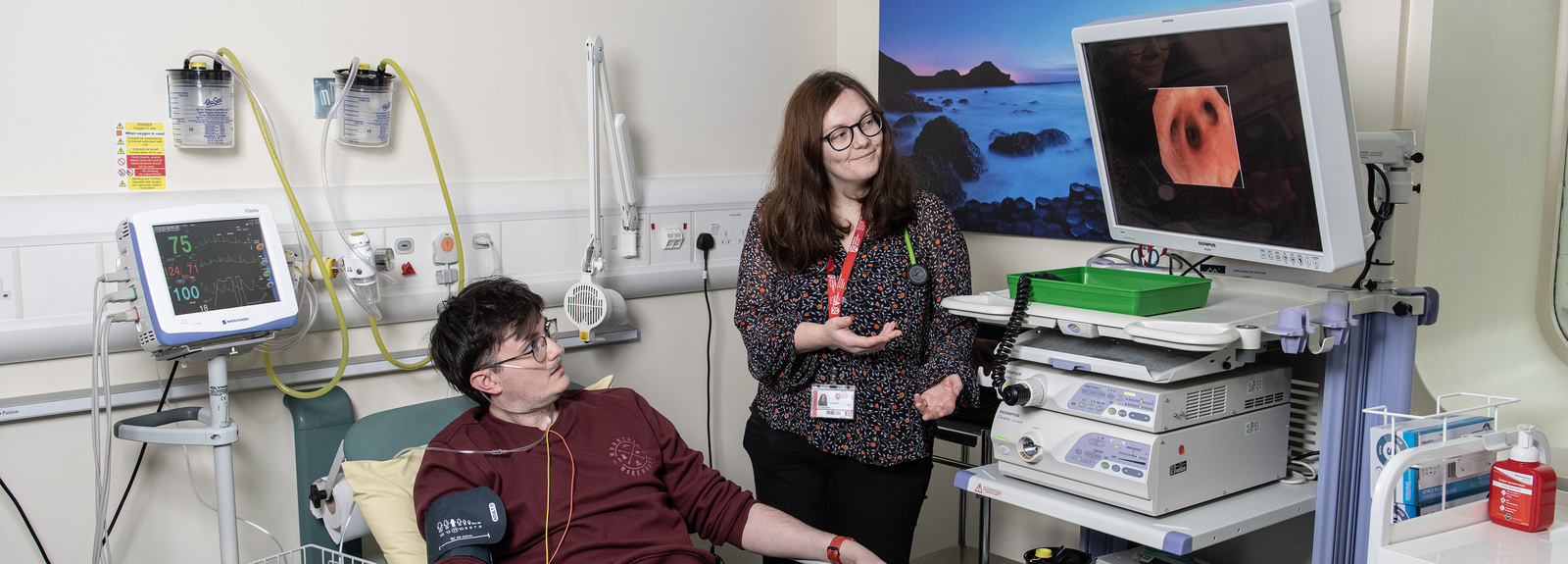
The Northern Ireland Clinical Research Facility (NICRF)
Established in 2013 with funding from the Wellcome Trust and Wolfson Foundation, the NICRF provides state-of-the-art facilities - supporting clinical research. The NICRF is a joint venture with Queen's University, Ulster University, Public Health Agency, Belfast Health and Social Care Trust.
NICRF Services
The Northern Ireland Clinical Research Facility (NICRF) supports clinical and translational research by providing state-of-the-art facilities and specialist services to researchers conducting health-related studies. It enables clinical trials, experimental medicine, and observational studies to be carried out in a safe and professional environment.
Explore our services below to discover how we can support your research.
-
NICRF General Clinical Support Services
- Blood sampling
- Blood sample processing
- Blood Sugar monitoring
- Cannulation and IV infusion
- Collection medication from Pharmacy
- ECGs
- Questionnaire administration
- Overnight observation
- Vital signs
- Walking tests
- Multiple Breath Washout
- Spirometry
- FeNO
- Bronchoscopy recovery
- Nutrition
- Bodystat
- Oral, Sub cutaneous and Intravenous and inhaled administration of licenced and unlicensed medications
- Mannitol challenge testing
- Transfer Factor test
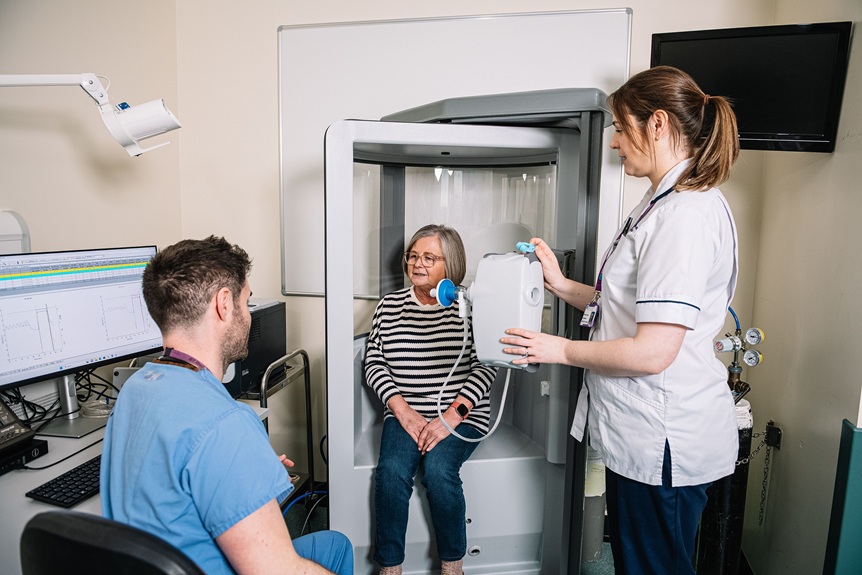
- Lung Volumes via body plethysmography (body box)
- Sweat Testing
- Nasal secretion testing
- Cough monitoring (VitaloJak and Digital Cough Monitors)
- Non-invasive ventilation - AIRVO
- NICRF Specialist Pulmonary Function Support Services
- Spirometry
- Lung Volumes via body plethysmography (body box)
- Respiratory muscle pressures (MIP/MEP)
- DLCO
- N2 washout
- Cardiopulmonary Exercise testing
- NICRF Optometry / Vision Science Support Services
Visual Function
- Refraction and Best Corrected Visual Acuity (BCVA)
- Low luminance and low contrast acuity
- Visual field assessment
- Macular assessment using microperimetry
- Dark adaptation measurement
- Electrophysiology of the eye
Ocular health examination
- Direct and indirect ophthalmoscopy
- IOP measurement
Ocular imaging
- Fundus photography
- Ultra-widefield imaging
- OCT/ OCT-A imaging
- Fundus autofluorescence
• Ocular biometry
- NICRF Laboratory Support Services
- Processing and short-term storage of biological samples - blood, stool, sweat, urine
- Packaging and transfer of samples
- Risk assessments / Standard Operating Procedures / User Guides
- NICRF Operational Support (Primarily available for studies using the NICRF) Services
- Liaison with Principal Investigators and Research Coordinators
- Study set-up
- Application process
- Costing Review
- Financial Tracking
- Invoicing
- Reception support
- Booking participants' appointments
- Room bookings
- Data and IT requirements
- Procurement, servicing and maintenance of equipment and resources
- Advertising new studies on the NICRF website/social media
-
NICRF Clinical Trial Practitioner Support
The tasks listed provide examples only rather than the exhaustive list of support services, staff are prepared to undertake additional training to meet service need of Principal Investigators.
- Site Qualification Visits / Feasibility Questionnaires
- Liaison with Principal Investigators and Research Coordinators
- Intensity Tools
- Local governance application
- Site File Maintenance
- Creation of study specific worksheets/source documentation
- EDGE
- Study Initiation
- Data Entry
- Support monitoring visits
- Close out / Archiving

Evolve Service Lab
Evolve is a GCLP-accredited clinical and translational research laboratory that offers bespoke services including;
- Central Laboratory Services
- Protocol Development
- Analytical Reporting
- Data Management Services
- Sample Management
-
Evolve Biomarker & Cell Signalling Analysis
- Profiling protein biomarkers and cell signalling pathways
- Bioanalytical validation: development, optimisation, and validation of biomarkers & surrogate markers
- Evolve Quality & Compliance
- GCLP facility (early phase trials) undergoing ISO 15189 accreditation
- Participates in external quality assessment (EQA) schemes (e.g., GenQA, UKNEQAS)
- Internal audits on all systems
- External audits by independent third parties for ISO compliance
- Evolve Areas of Expertise and Sample Handling Capability
- Focus areas: Respiratory, Oncology, Dermatology, Infectious Diseases (bacterial, viral, fungal pathogens)
- Processing of multiple sample matrices, including:
- Sputum, blood, BAL, urine, bronchial/nasal brushes, nasal scrapes, saliva, stool
- Nasal and nasopharyngeal swabs, biopsies
- Laboratory techniques
- Flow cytometry (QUB facilities):
- Cell sorting, extracellular/intracellular fluorescent marker detection
- Immunophenotyping, cell cycle analysis, cell proliferation & death
- Imaging: Immunocytochemistry, primary & cell line cultures
- PBMC preparation (manual)
- Flow cytometry (QUB facilities):
- Microbiology & Clinical Services
- Bacterial culture, quantification, PCR-based bacterial/viral identification
- Susceptibility testing
- Clinical kitting services